Abstract
Introduction
Haemophilia is a pathology with a great impact on the sufferer and their environment, that is perceived in the family as an exceptional event, interferes with the daily lives of patients and caregivers, emotionally, occupationally and socially1. Joint damage remains a major complication associated with hemophilia that is widely accepted as one of the most debilitating symptoms and has a significant negative impact on health-related quality of live (HRQOL) for adults with severe hemophilia, who frequently experience anxiety and depression. The quality of life of caregivers of haemophilia patients is also affected by the disease2. This abstract presents the baseline results of a sample of patients and caregivers with severe haemophilia A participating in HEMOLIFE study related to anxiety and depression as measured by the Hospital Anxiety and Depression Scale (HADS).
Methods
Multicenter, prospective, observational study to assess changes in socio-occupational impact1 over a 12-month period in patients ≥ 12 years with moderate or severe haemophilia A without inhibitors and their caregivers (people who share the burden of the disease in the family environment). Information related to depression and anxiety has been collected using the HADS questionnaire3,4. HADS is a 14-item scale with seven items each for anxiety and depression subscales. Patients and caregivers completed the HADS via the study App.
Results
The study included 81 patients with a mean age of 33 years (81.5% adults), 40% with a partner, 46% workers and 34% students. Forty-seven percent of the patients had comorbidities, the most frequent being haemophilic arthropathy (12%), HIV (10%) and arterial hypertension (10%), hepatitis C (9%), obesity (5%), hypercholesteremia (5%) and diabetes mellitus (5%). In terms of severity, 83% had severe haemophilia. All patients were receiving prophylactic treatment with plasma or recombinant factor VIII (51%), extended half-life factor VIII (31%) and emicizumab (18%), and in 80% the regimen was more than once a week. Twelve caregivers were included in the study with a mean age of 48 years, most of them were women (83.3.%), without university degree (91.7%) and married or with partner (83.3.%); 33.3% was unemployed. Most caregivers have 1 child with hemophilia (75.0%) and 87,5% had at least 2 children. The mother was the caregiver in half of patients, in 33.3% of the cases it was the partner and in 16.7% the father. Mean time caring for person with hemophilia was 19.5 years.
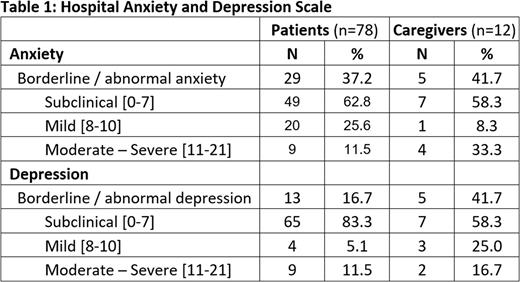
Of the 81 patients included in the study 78 completed the HADS and all 12 caregivers included in the study completed the HADS. In the patients the mean (standard deviation) score of the anxiety scale was 6.2 (4.3) and of the depression scale 4.2 (4.5). In caregivers the score on the anxiety scale was 8.0 (5.4) and on the depression scale 5.8 (4.0), in both cases higher than in patients. Percentage of subjects in the category borderline / abnormal for anxiety and depression was higher in caregivers than in patients: 41.7% vs 37.2% and 41.7% vs 16.7% respectively. Results are shown in table 1.
Conclusions
Depression and anxiety are two important issues to consider in the haemophilia patient environment, both for the patient and the caregiver5. The results of this analysis seem to suggest that caregivers are more affected than patients in these two aspects. Depression and anxiety are two relevant aspects in the environment of haemophilia patients and their presence should be regularly assessed in both patients and caregivers in order to take measures to avoid unfavorable evolution.
Disclosures
Alvarez Román:Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other; Novo Nordisk: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia; Octapharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia; Grifols: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia; Roche: Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia; Biomarin: Consultancy, Honoraria, Research Funding; Sobi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board ; CSL-Behring: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia. Guerra:Roche Farma Spain: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal